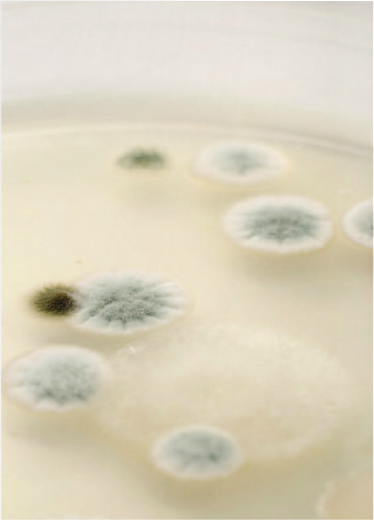
Key Considerations for Successful Preservation of Cosmetic Products: PART ONE
In this first part of this two-part article, we aim to answer common preservative questions and to dispel some common myths. We will also examine self-preserving cosmetics and preservation guidelines in Europe and the USA. Spoilage can be caused by bacteria, moulds or sometimes yeasts growing in products such as creams, lotions and shampoo during product storage. These microbes entered the product owing to contaminated raw materials, poor preservation or poor hygiene practice during manufacture. Some types of microbes can cause infection and disease, and often no visible spoilage is evident. Organisms such as Staphylococcus aureus can cause skin infections. Pseudomonas aeruginosa can cause very serious eye infections and Candida albicans (Yeast) can cause ‘thrush.
Product types vary in their susceptibility to contamination and growth of microbes. The most susceptible products are those which contain water, such as creams, shampoo, shower gel, lotions and hair conditioners.An effective preservative system is therefore needed for consumer protection and prevention from spoilage during normal product use.
Let’s look at a few of the common questions regarding preservatives. I made a lotion and did not add a preservative.
How long can I use it for? This will vary according to the formula, packaging and other considerations. Perhaps anywhere from
0 to 7 days from making it, if stored in the fridge.
Do lip balms, balms and body butters which do not contain water need a preservative?
Generally they do not; however, if water is likely to enter the product-for example, from a humid bathroom, a preservative is advisable.
It is important to note that aloe vera, goafs milk, hydrosols and floral waters consist of mainly water and are challenging to preserve. What guidelines does the FDA (USA) and the EU (Europe) provide regarding preservation? In the EU, the EU Cosmetics Directive (“the Directive”? requires that cosmetic products be safe under normal or reasonably foreseeable conditions of use. In order to demonstrate that a cosmetic product complies with the Directive, the responsible person shall, prior to placing a cosmetic product on the market, ensure that the cosmetic product has undergone a safety
assessment. Annex I of the Directive states that the Cosmetic Product Safety Report shall as a minimum, contain the results of the preservation challenge test and an assessment as to its safety.